Abstract
Introduction: Mutations in TP53 confer an adverse prognosis in patients (pts) with AML and HR-MDS. There are conflicting results regarding the prognostic impact of mono-allelic vs bi-allelic and mono-hit vs multi-hit TP53 mutation status on prognosis in AML and HR-MDS. Additionally, assessment of loss-of-heterozygosity (LOH) status is not routinely available in many settings limiting the clinical implementation of TP53 mutational status.
Methods: We took advantage of a large, randomized study with extensive clinical and molecular annotation to analyze 61 patients (pts) with TP53-mutated (TP53-mut) disease and to compare them to 144 TP53 wild-type pts. All pts received azacitidine and half were randomized to receive the anti-PDL1 antibody durvalumab, which did not improve clinical outcomes (Zeidan et al. Blood Advances 2022). A 38 gene-targeted myeloid mutation analysis from screening bone marrow was performed at Munich Leukemia Laboratory. Only pathogenic TP53 variants with variant allele frequency (VAF) ≥2% were included. Pts with a common SNP or VUS in the TP53 gene were classified as TP53-WT in the analysis. Multi-hit TP53 mutations were defined as previously published by the International Consensus Conference (ICC; Arber et al. Blood 2022) as ≥2 distinct TP53 mutations (VAF ≥10%) or a single TP53 mutation associated with either: 1) cytogenetic deletion involving chromosome 17p (e.g., del(17p) or monosomy 17); 2) a VAF of >50%; or 3) with any complex karyotype. We also defined multi-hit TP53 mutations as proposed by Grob et al. (Blood 2022) as (I) ≥2 TP53 gene variants irrespective of VAF, (II) ≥1 TP53 gene variant co-occurred with a cytogenetic deletion involving chromosome 17p, or (III) TP53 mutations with VAF >55%. Based on multiple prior studies (Grob et al. Blood 2022, Weinberg et al. Blood Advances 2022) patients with AML and HR-MDS with TP53 mutations were analyzed as one disease entity.
Results: 37 had TP53-mut AML, 89 were TP53-WT AML, 24 had TP53-mut HR-MDS, and 55 were TP53-WT HR-MDS pts. The average VAF for TP53 mutations was 37%, and 90% had ≥10% VAF. 93% of TP53 mutations occurred in the p53 DNA-binding domain. TP53-mut pts had fewer co-occurring mutations compared with TP53-WT pts (mean number of mutations: 1.8 [SD: 0.77] for TP53-mut pts (including TP53) vs 2.9 [SD: 1.4] for TP53-WT pts; Wilcoxon P <0.001) with distinct patterns in the distribution of co-occurring mutations. In terms of TP53 allelic status, 22 (11.0%) were mono-hit and 41 (20.5%) pts were multi-hit as defined by Grob et al, while 7 (3.4%) and 56 (28.0%) pts were classified as mono-hit and multi-hit per ICC definition, respectively.
In the AML cohort, TP53-mut pts were more likely to have poor-risk cytogenetics (59.5% vs 11.2%; p<0.001), therapy-related myeloid neoplasm (10.8% vs 1.1%; p=0.042), and lower bone marrow blast percentage (27.5% vs 35.5%; p=0.001) compared to TP53-WT. Among MDS pts, TP53-mut pts were more likely to have secondary MDS (20.8% vs 1.8%; p=0.013), very poor risk cytogenetics by IPSS-R (75% vs 14.6%; p<0.001), and very high risk IPSS-R score (62.5% vs 34.6%; p=0.039). There were no statistically significant differences in terms of baseline demographics and other clinical characteristics.
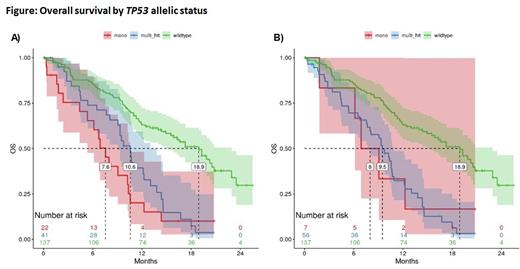
There was no difference in overall response rate (ORR) between TP53-mut and TP53-WT pts (AML: ORR: 35.1% [95% CI: 20.7% - 52.6%] vs. 33.7% [95% CI: 24.2% - 44.6%]; p=1.000; MDS: ORR: 41.7% [95% CI: 22.8% - 63.1%] vs. 60.0% [95% CI: 45.9% - 72.7%]; p=0.208). TP53 allelic status did not influence OS using either definition (Grob et al: mono-hit: median OS 7.6 months [95% CI:5.4-10.6 months]; multi-hit 10.6 months [95% CI: 9.1-13.0 months] (Figure A); ICC definition: 8.0 months (95% CI: 6.1-20+ months) and 9.5 months [95% CI: 7.7-10.9 months], (Figure B)) and was inferior to TP53-WT (median OS: 18.9 months; 95% CI: 15.5-22.9 months).
Conclusion: Outcomes of AML and MDS pts with TP53 mutations are worse compared to TP53-WT without differences between mono-hit vs multi-hit status as defined by the ICC and Grob et al. This suggests that either the negative prognostic impact of a multi-hit TP53 allelic status can be overcome by treatment with azacitidine or that a distinction by TP53 allelic status in newly diagnosed AML/HR-MDS pts treated with azacitidine does not provide additional prognostic information. Whether incorporating TP53 LOH status is necessary to further refine risk assessment warrants additional studies.
Disclosures
Hasle:Bristol Myers Squibb: Current Employment. Shallis:Bristol Myers Squibb and Gilead Sciences, Inc: Honoraria; Gilead Sciences, Inc.: Honoraria. Thompson:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Lopes de Menezes:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Rose:Bristol Myers Squibb: Current Employment. Boss:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Halene:Forma Therapeutics: Consultancy. Haferlach:Munich Leukemia Laboratory: Current Employment, Other: Part ownership. Fox:Bristol Myers Squibb: Current Employment. Zeidan:Novartis, Cardiff Oncology, Pfizer: Other: Travel Support; Celgene/BMS, Novartis, AbbVie, Gilead, Kura, Loxo Oncology, Geron: Other: Clinical Trial Committee; Gilead, Kura, Loxo Oncology: Consultancy, Honoraria, Other: Clinical Trial Committee; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie, Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Astex, Pfizer, Medimmune/AstraZeneca, ADC Therapeutics: Research Funding; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie: Consultancy, Honoraria, Other: Advisory Board; Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Gilead, Kura, Loxo Oncology, Otsuka, Jazz, Agios, Acceleron, Astellas, Daiichi-Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Ionis, Epizyme, Janssen, Syndax, Genentec: Consultancy, Honoraria, Other: Advisory Boards; Astex, Medimmune, Astrazeneca, ADC Therapeutics: Research Funding; Celgene/BMS, AbbVie, Pfizer, Boeringer-Ingelheim, Trovagene, Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, Amgen, Otsuka: Consultancy, Honoraria, Research Funding; Jazz, Agios, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, Beyondspring, Gilead, Kura, Tyme, Janssen, Syndax, Geron, Ionis, Epizyme: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal